A longitudinal cohort study of early brain development and motor skills in typically developing infants and infants at risk for cerebral palsy aged 3-24 months.
Cerebral palsy (CP) is the most common severe motor disability in children, often resulting from non-progressive brain injury or malformation that occurs during early brain development. Early diagnosis is crucial for initiating targeted treatment, as the brain is more plastic at a younger age. Today, diagnostic MRI of infants with suspected brain damage mainly relies on conventional structural MR scans, which focus on identifying major structural brain pathology. However, advanced MRI methods, such as multi-shell diffusion imaging, may provide deeper insights into subtle neuropathological changes. MRI of infants and toddlers is typically conducted using sedation or general anesthesia (GA). Yet, concern has been raised regarding the potential neurotoxic effects of GA, especially for developing brains.
The NIBS-CP Cohort
The NIBS-CP study will follow approximately 200 infants, including both those at high risk for cerebral palsy (recruited through the Danish national Cerebral Palsy: Early Diagnosis and Intervention Trial (CP-EDIT)) and typically developing infants. Participants and their parents are invited to three study visits: at inclusion (3-9 months of age), at 12 months, and again at 24 months. At each visit, the children undergo MRI scanning as well as motor and neurological assessments (see figure below for the study outline).
NIBS-CP aims and objectives
NIBS-CP has several key objectives that focus on improving diagnosis and understanding of early brain development in infants and toddlers with CP and other neurological disorders that require cerebral MRI. The project has four main objectives:
- Establish safe infant MRI without anesthesia - Develop procedures for clinical MRI during natural sleep, minimizing sedation or general anesthesia at Hvidovre Hospital and the future Mary Elizabeth’s Children’s Hospita
- Implement advanced MRI methods - Use techniques such as diffusion imaging that may be more sensitive for detecting subtle brain injury than conventional MRI.
- Characterize early brain development - Investigate how brain development differs in infants at risk for CP compared to typically developing infants, using “growth chart-like” models to track typical and atypical trajectories.
- Link early brain and motor development - Map relationships between brain structure and motor function to identify imaging features that may enable a better prediction of the child's motor function and motor development prospects.
Ultimately, NIBS-CP aims to lower the average age of CP diagnosis in Denmark, ensuring that children receive the support and interventions they need as early as possible.
NIBS-CP Collaboration
NIBS-CP runs from 2022 to 2027 and brings together expertise across disciplines and institutions. The study is a collaboration between the DRCMR, the Department of Physio- and Occupational Therapy at Hvidovre Hospital, the Department of Computer Science at the University of Copenhagen, and the CP-EDIT study led by Prof. Christina Høi-Hansen at Rigshospitalet. The project will also contribute to establishing clinical infant MRI procedures at Hvidovre Hospital and the future Mary Elizabeth’s Children’s Hospital.
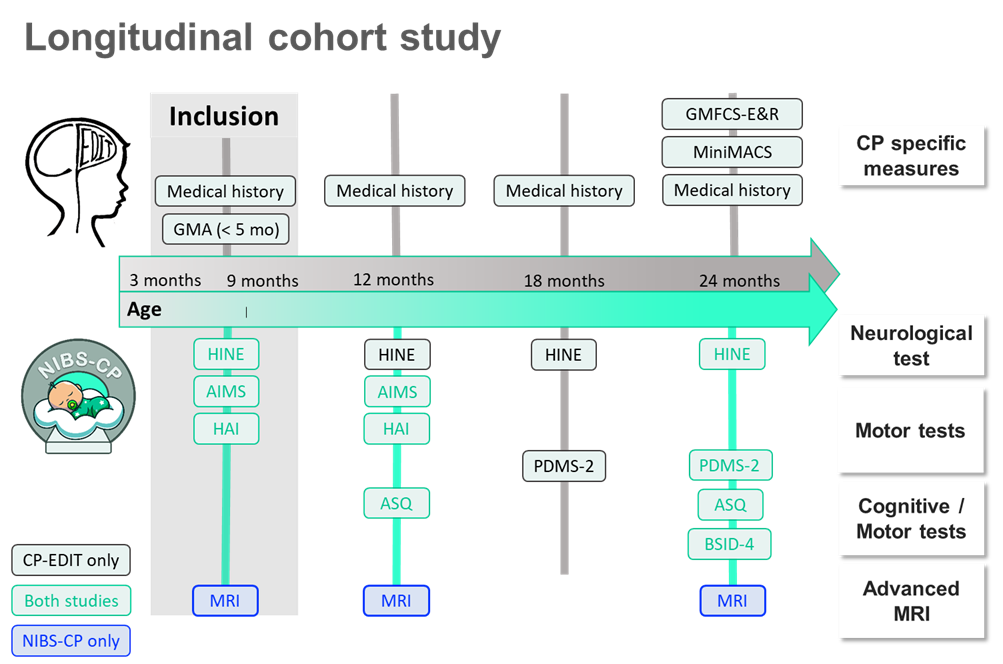
Outline of the NIBS-CP study and CP-EDIT. All children will undergo MRI scans three times over a 2-year-period, from ages 3 to 24 months. At baseline (age 3-9 months), children will be assessed with the Hammersmith Infant Neurological Examination (HINE), the Hand Assessments for Infants (HAI), and the Alberta Infant Motor Scale (AIMS). At the first follow-up (age 11-12 months), the children will be re-assessed with AIMS and HAI, as well as with the Ages & Stages Questionnaire (ASQ). Additionally, children at risk of CP will be re-assessed with the HINE. At the second follow-up (age 24 months), all children will be assessed with HINE, Peabody Developmental Motor Scales (PDMS-2), ASQ, and the Bayley Scales of Infant and Toddler Development 4th Edition (BSID-IV).







