The Movement Disorders group is headed by David Meder. Our research primarily focuses on Parkinson´s disease (PD), but also REM-sleep behaviour disorder and progressive supranuclear palsy.
The mission of the Movement Disorders group is to use advanced brain mapping techniques in combination with computational modelling to investigate how movement disorders alter brain function and structure in motor, cognitive and limbic systems. We use the ultra-high field (7 tesla) MR scanner in order to map the structural integrity of midbrain nuclei at high resolution. This allows us to investigate the relationship between the individual spatial pattern of neurodegeneration in Parkinson’s disease, the resulting changes in brain function and finally the patient’s clinical symptoms.
We apply computational models of learning and decision-making to probe disease-induced changes in brain and behaviour. Traditionally, clinical neuroimaging has often taken a predominantly descriptive approach, describing that there is a change in brain or behaviour observed in the disorder. Computational neurology goes one step further: We create computer algorithms that mimic how the function under investigation might be solved by the brain. If such a model then fits to the observed behaviour and neural activity, we can infer that the brain (approximately) uses the kind of algorithm we defined in the model. Observing which parameters of the model are changed in the disease can then lead to a mechanistic understanding, explaining not only that there are disease-induced changes, but how the changes occur at the neural and symptomatic level.
Furthermore, a large part of our efforts revolves around the use of transcranial magnetic brain stimulation (TMS). TMS is used both to explore the excitability of the motor system, but also as a precision treatment approach advancing personalized medicine.
Key Projects
ADAPT-PD
Many members of the Movement Disorders group are part of the ADAPT-PD project. Here, we investigate dysfunctional circuit dynamics in cortico-basal ganglia projections in PD with the aim to "normalize" them with brain stimulation techniques. ADAPT-PD is a collaborative international, translational and multi-modal project involving different techniques (invasive and non-invasive recordings as well as brain stimulation) in animal models and PD patients. Detailed information can be found here: ADAPT-PD.
Unravelling altered network dynamics in the mid-brain and striatum in Parkinson's disease with ultra-high field MRI
In this project, we use ultra-high field (7T) functional and structural MRI to map the individual degree and spatial pattern of neurodegeneration in PD patients and relate it to the patient’s motor and non-motor symptoms. We focus on two neurotransmitter systems, the dopaminergic and the noradrenergic system.
The large majority of dopamine neurons are situated in two midbrain nuclei, the ventral tegmental area (VTA) and the substantia nigra pars compacta (SNc). Using high resolution imaging, we can show that dopaminergic neuron loss and iron accumulation in the SNc show an overlapping, but also spatially distinct disease pattern with highest disease sensitivity in the nigrosomes 1, 2 and 4 (Madelung et al. 2025).
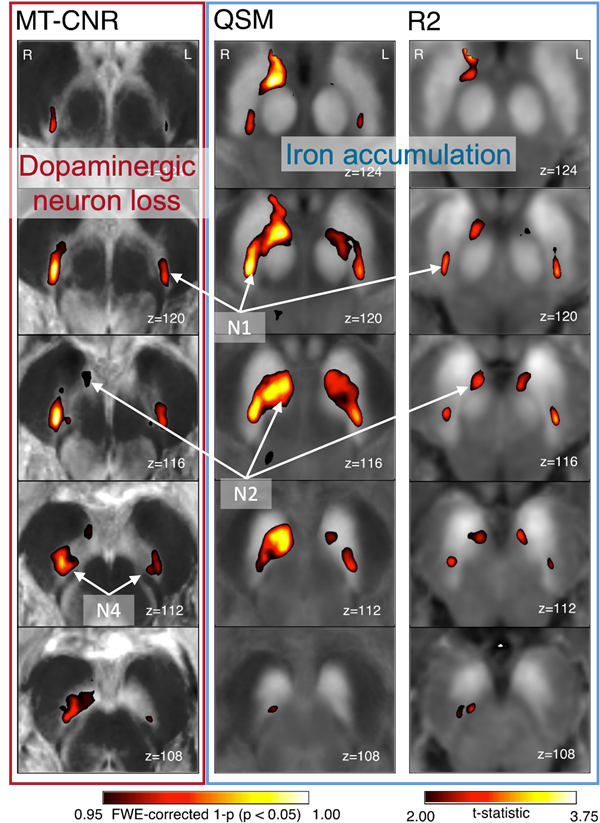
While the neurodegeneration in the SNc is mostly responsible for the motor symptoms in PD, VTA neurons are associated with reward-based learning. Here, we interrogate the relation between the neurodegeneration in these regions measured with structural MRI and the functional activation during a task that requires learning about the values of certain actions and stimuli.
The locus coeruleus (LC), a small nucleus in the brain stem, is the main source of noradrenergic projection neurons and it is also heavily affected by neurodegeneration in PD. Noradrenaline lies at the core of eliciting an arousal response to novel and surprising events and has a tight coupling to the sympathetic nervous system. We can show that the LC does not degenerate uniformly and that cell death in different parts of the structure correlate with different non-motor symptoms (Madelung et al. 2022).
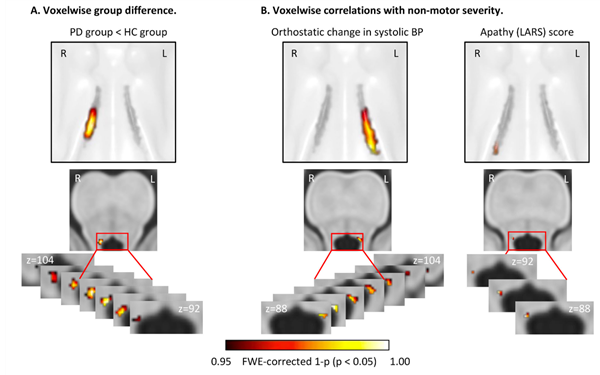
We furthermore acquire functional images of the LC while participants are presented with surprising and emotionally arousing auditory and visual stimuli which we then relate to the individual degree of structural disintegration.
RBD project
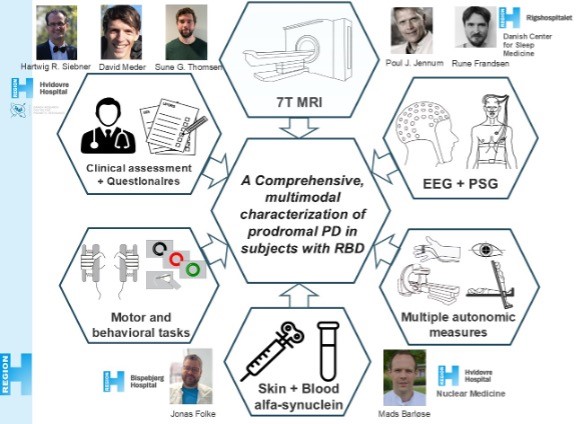
In an upstarting project, we extend the structural imaging to a cohort of patients with REM-sleep behaviour disorder (RBD). Patients with this disorder have a high probability of developing an alpha-synucleopathy such as Parkinson’s disease, Lewy-body dementia or multiple system atrophy. Since the conversion rate to PD is considerably higher than the other synucleinopathies, RBD is often considered a prodromal state of PD. In this collaborative project, we aim to achieve a comprehensive characterization of these patients, combining our structural ultra-high field scanning with neuropsychological and motor performance measures, detailed sleep assessment with polysomnography from the Danish Center for Sleep Medicine at Rigshospitalet Glostrup, a thorough assessment of the autonomic nervous system at the Department for Nuclear Medicine at Hvidovre Hospital and finally measures of alphy-synuclein via blood-samples and skin biopsies.
OPD
In the OPD project (Optimism and Pessimism in the Dopamine System in PD), we test a novel theory suggesting a hitherto unknown diversity in dopaminergic signalling. The main aim is to investigate whether the healthy human brain shows a diversity of optimistic and pessimistic reward signals and whether changes in this distribution in PD can provide mechanistic insights into the cause of symptoms. In order to address these questions, we will apply a unique combination of theory and methods on multimodal imaging and behavioural data acquired in healthy participants and PD patients. Using the fMRI signal in dopaminergic regions as proxy for regional dopaminergic signalling, we will probe reward and movement related activity in VTA/substantia nigra pars compacta (SNc) and striatum with functional magnetic resonance imaging (fMRI) at 3 tesla (3T). We will derive functional topographic maps of optimism/pessimism by applying a novel neuroimaging analysis method to the fMRI data developed at the department. Observing which parameters of the model are changed in the disease can then lead to a mechanistic understanding of how the disease affects brain function, leading to different symptom.






