Our mission is to identify metabolic markers of incipient brain alterations that likely precede microstructural and structural brain impairments utilizing state-of-the-art high-field (3T) and ultrahigh-field (7T) scanners. Novel markers will enable the identification of ongoing brain deficits in various neurological and psychiatric disorders, track treatment effects, and are therefore urgently needed for larger clinical trials. Motivated by the desire to identify quantifiable markers of neuronal deficits, we utilize and develop innovative methods for dynamic spectroscopic measurements, such as deuterium metabolic imaging (DMI) and functional spectroscopy, to track abnormalities in neuro-metabolism, including glucose metabolism and its downstream processes. The regional impairment of the brain´s glucose metabolism plays a crucial role in the pathophysiology of severe brain conditions such as major depressive disorder and Alzheimer's disease.
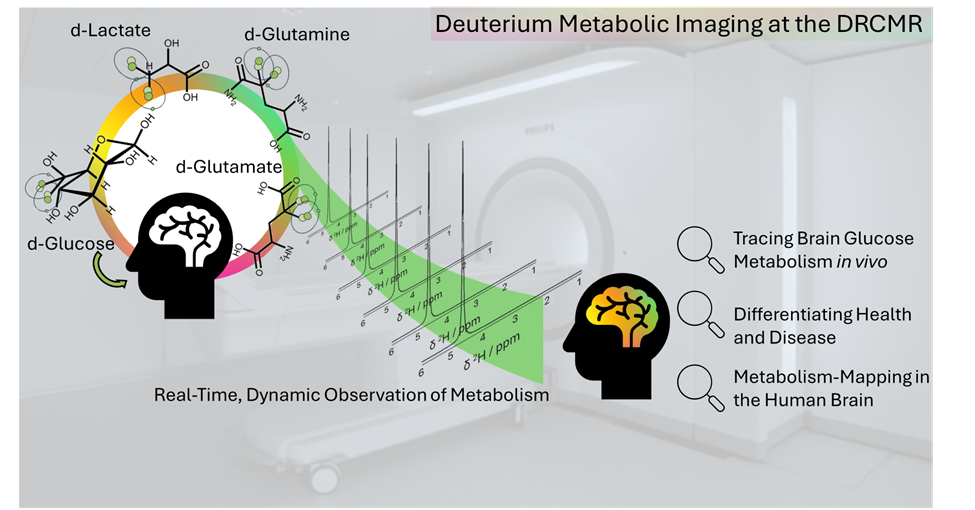
Deuterium metabolic imaging (DMI)
Sensitive and non-harmful methods using stable, non-harmful tracers are urgently needed to capture key aspects of Glucose downstream brain metabolism in vivo, without the need for extensive scanning hardware. DMI holds great promise to match this need. Deuterated Glc enters the oxidative Glc pathway and is incorporated into downstream metabolites and tricarboxylic acid Krebs cycle in mitochondria, and thus allows for the separate quantification of anaerobic, Krebs, and Glu/glutamine (Gln) paths, while addressing the downsides of the current gold standard for metabolic measures, i.e., positron emission tomography.
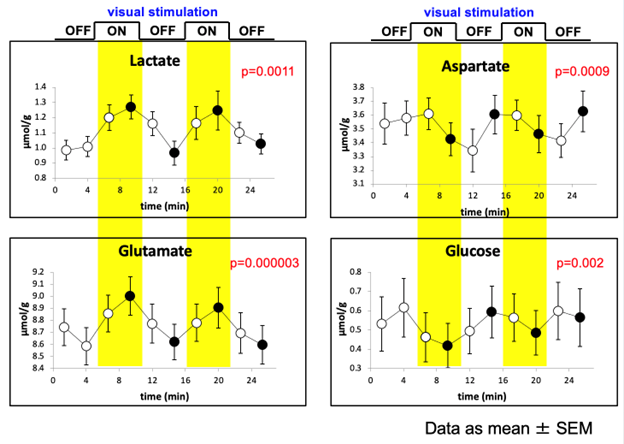
Functional spectroscopy
Proton functional magnetic resonance spectroscopy (¹H-fMRS) is an MR spectroscopy technique that measures task- or stimulus-related changes in the concentrations of brain metabolites in vivo over time. Unlike conventional (static) ¹H-MRS, which provides a snapshot of baseline metabolite levels, ¹H-fMRS tracks dynamic fluctuations — for example, increases in glutamate or lactate during neuronal activation, or modulation of GABA during inhibitory processing. This allows probing the neurochemical underpinnings of brain function and complements hemodynamic measures such as fMRI.