Vision
Our vision is to integrate basic research with human research in a translational forward (rodent-to-human) and backward (human-to-rodent) approach with the ultimate goal to improve the treatment and diagnosis of brain disorders. Our research spans multi-modal microstructural, functional and metabolic imaging in combination with brain stimulation and other interventions with potential therapeutic relevance.
Aim
To facilitate users with state-of-the-art imaging technologies and equipment that are centred around MRI to enable multimodal integration of brain micro-structure, functional and metabolic information.
Facilities
The scanner room environment is designed to run both technological driven projects as well and in vivo animal neuroscience projects:
- GMO class II lab ("virus lab")
Animal Housing
Animal facilities for rodents (rat and mice). This is our main stable where most of the work with our different animal models are done. The room is temperature, humidity and light (12:12) regulated to ensure that the animals have the optimal living conditions.
Equipment:
2 scantainers - here we house our rats and mice
Dishwasher - to wash dirty cages and appliances
Change station - to empty dirty sawdust
GMdyr Main Lab
Histology:
For preparing tissue (brain tissue that has been perfusion fixated) we have borrowed a Vibratrome from our collaborator Bente Pakkenberg at N-Lab (Bispebjerg Hospital). This can be used for investigating the tissue for viral expression, tracers, etc.

Minor surgeries:
Minor surgeries - for instance be PostOp Treatment, Peripheral Venous Catheters, and other procedures for preparing the animals for experiments (that do not require a GMO class II facility) - can be performed in the main lab. Available at the station is Isoflurane anaesthesia, a light stereomicroscope and basic surgery equipment.
Electronics and electrode + optic fiber production
A small work bench for producing microwire tetrodes, microdrives and optic fiber implants is available. Soldering and electronic test equipment is available.
Electrophysiology recording setup:
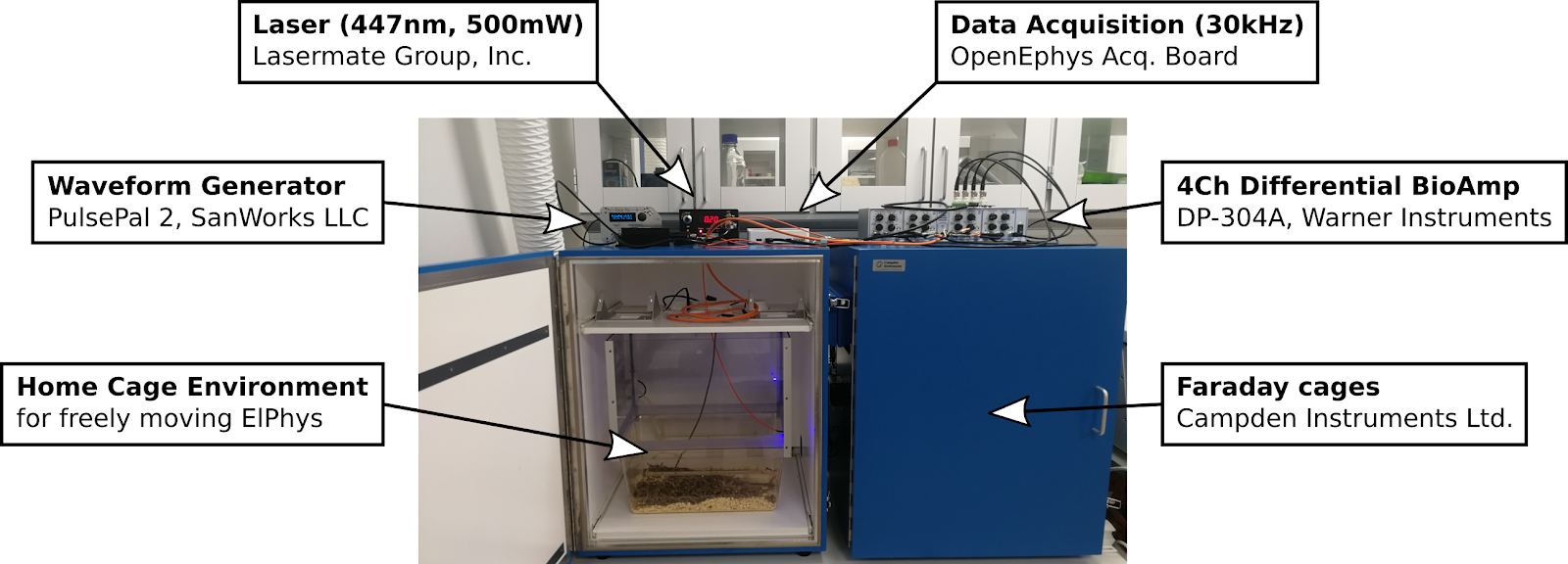
Bio Amplifiers:
- Intan RHD2132 (32 channels)
- Warner Instruments DP-304A (4 channels)
Data Acquisition:
- OpenEphys Acquisition Board (OpenEphys.org)
Waveform Generator (Pulse Generator):
- PulsePal rev.2 (SanWorks LLC)
Laser:
- 447nm, 500mW (LaserMate Group, Inc)
Linear Stimulus Isolator:
- WPI A395RC (World Precision Instruments)
Available for use with both freely moving ElPhys (commutators and RatCageExtender available) and for anesthetized experiments.
GMO class II lab (“virus lab”)
In the preclinical lab area (all classified for working the genetically modified animals “GM animals”) we also have a dedicated lab room that is classified for working with genetically modified organisms (GMO) class II (e.g. AAV, CAV and HSV). It has a small sluice area, in which the personnel add extra personal protective clothing.
In the lab is also a small desktop autoclave (Systec DE-45) for sterilizing surgery equipment as well as stuff that needs to leave the room at some point. The room has two separate scantainers for housing animals, each with a capacity of 5 singly housed animals (e.g. 2*5 rats or 5mice and 5rats). The lab has a LAF bench for specifically working with the GMOs and for perfusion fixation of animals.
The scanner room
We have a Bruker 7T Biospec MRI scanner at our facilites. The MR scanner is based on an Ultra Shielded and Refrigerated (USR) magnet with bore size of 20 cm and is equipped with a main gradient set of 660 mT/m with inner diameter (ID) of 114 mm. Additionally a gradient insert of 1000 mT/m (ID:xx ) is available for e.g. diffusion MRI. RF receive is a four parallel channel system for proton with a single broad-band RF transmit/receive channel for X-nuclei.
RF coils are an important factor to ensure good image quality as a treat off between image resolution and signal-to-noise ratio (SNR). We have a wide range of RF surface, quadrature and parallel receive/transmit coils. They exist in various sizes for imaging applications ranging from small ex vivo tissue samples to in vivo rodent.
The system is equipped with 2-channel cryo-coil transmit/receive RF coil for in vivo mice imaging or post-mortem imaging of small tissue samples. The cryo-coil design reduces thermal noise in the electronics of the RF coil and can increase the SNR by a factor of 2-5.
The Paravision 6.0 software is a visually easy-to-use interface for setting up the MRI protocols and to run scanning. It enables advanced access to control scan parameters not normally available on clinical systems. We hold the expertise to develop new MRI sequence designs. Especially within diffusion MRI for both in vivo and ex vivo imaging i.e. microstructure imaging and have established online BOLD functional MRI and simultaneous optogenetic brain stimulation opto-fMRI.
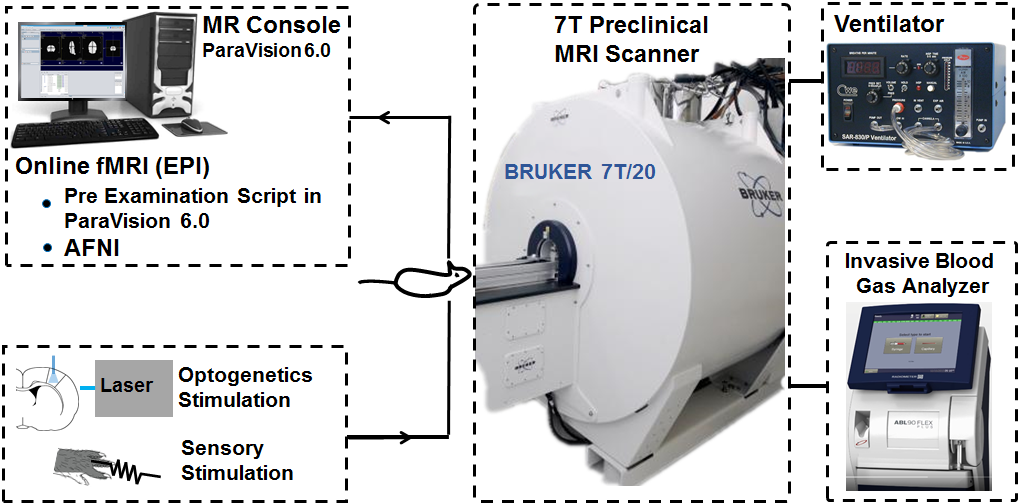
Online fMRI with Sensory or Optogenetics Stimulation
Hardware lab
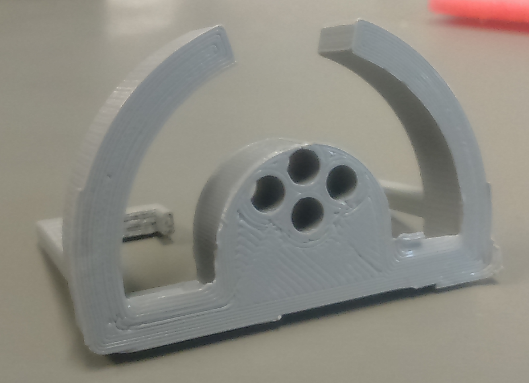
The preclinical group can access a 3D printer (‘’Ultimaker 2 Extended’’), with a build volume of 223 x 223 x 305 mm, to create custom-designed objects to help and improve the experimental setup construction. The group primary uses black PLA (InnoFil Pro1) filament, but different colors and materials are available upon request.

3D printer (‘’Ultimaker 2 Extended’’), with a build volume of 223 x 223 x 305 mm
Figure of sample holder (custom made 3D-model) for small tissue samples. Printed on our 3D-printer:
Please find more info on the Hardware lab.





